Potential Protein Drugs are Urgently Needed to Treat Pulmonary Fibrosis
Pulmo BioMed, established by JOYCOM Group in the year 2021, is a biotechnology pharmaceutical company focusing on new drug development. Dr. Le-Shin Chang serves as the head of the R&D team and the president of Pulmo BioMed. With the professional pharmacology background, Dr. Chang has been working in the biotechnology and pharmaceutical industry for more than 20 years. In addition, she has extensive experience on researching & developing, and currently obtained 20 domestic and foreign patents including Taiwan, USA, Germany, China, Japan and so on. She has led the development of medical formulation for years and completed developing several medical products. The medical product focusing on wound repair “NewEpi®” developed by Dr. Chang won the 29th Taiwan Excellence Awards. It is granted by all major hospitals and has been widely used on the wound healing of surgery, burn and skin grafting, etc. Another medical product - Vaginne® Intimate Gel offers a complete care of intimate part and provides every woman a fully experience of natural fresh and intrinsic comfort. The precious patent Vaginne® contained in the products not only suppresses pathogenic bacteria but also promotes the growth of beneficial flora. Vaginne® Intimate Gel has been popularized in Taiwan and other countries and receives critical acclaim worldwide.
Link:JOYCOM Group
As many human diseases do not yet have appropriate medications, there is still lots of room for drug development. Dr. Chang leads the R&D team to invest in the field of drug research and development. The R&D group of Pulmo BioMed has been deeply working in the field of protein/peptide function for many years. Now we have successfully developed a new protein drug – PulmoHeal® with three advantages: (1) reversal of pulmonary fibrosis (2) short‐term therapy (3) high safety. PulmoHeal® can effectively reverse pulmonary fibrosis. The research and development achievements of PulmoHeal® were a breakthrough therapy in the treatment of pulmonary fibrosis. Because PulmoHeal® has a great development potential, we officially established Pulmo BioMed Co., Ltd. in 2021 to focus on recruiting funds and accelerating the acquisition of drug license. At the same time, we commit to promoting the listing of the company, optimize corporate governance levels and reinforce the management.
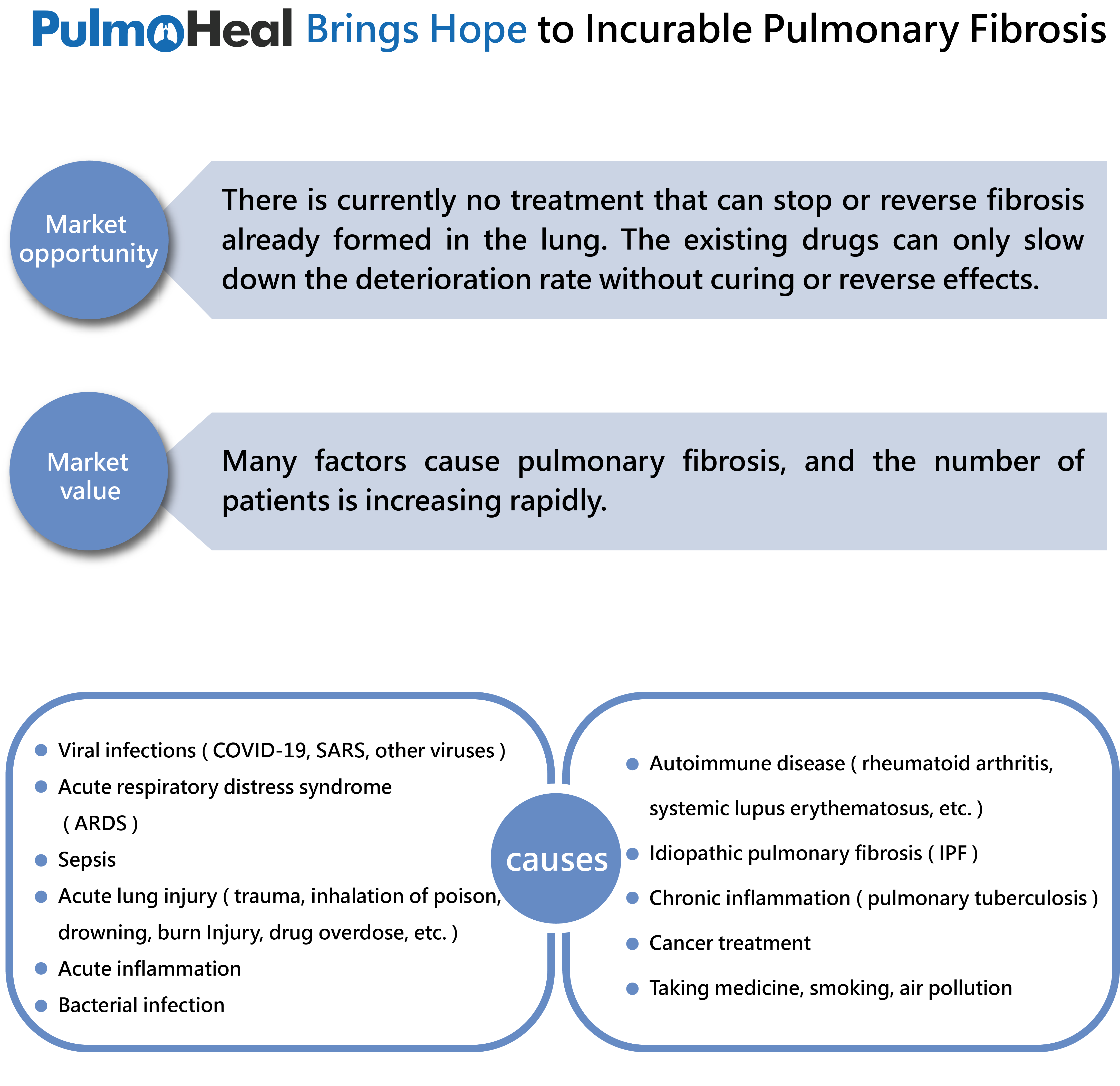
Many reasons cause pulmonary fibrosis, such as virus infection (COVID-19, SARS and other viruses), IPF, autoimmune disease (rheumatoid arthritis, lupus erythematosus, etc.), chronic inflammation, radiation therapy, cigarette smoking, air pollution and so on. Pulmonary fibrosis causes dyspnea and progressive deterioration of lung function in patients with a poor prognosis. Life expectancy of people with pulmonary fibrosis is generally less than five years. Worldwide, the incidence and prevalence of pulmonary fibrosis are increasing. Taking interstitial lung disease (ILD) for example, the prevalence of ILD are estimated 76.0 per 100,000 people in Europe and 74.3 per 100,000 people in the USA. According to research, patients with ILD have a high probability of developing pulmonary fibrosis. In addition, there are about 3 million people with IPF (a specific form of chronic, progressive fibrosing interstitial pneumonia) in the world and the number of patients is increasing rapidly. The global markets forecasting of IPF will reach a value of $8.8 billion by 2027. This reflects that the large patient population makes it become a hot treatment market and the demand is urgent. However, there is no effective cure for IPF. Currently, clinically approved drugs are chemical drugs which have long course of treatment and can only slow down the deterioration rate without curing effects. Patients with poor liver function must be monitored or even cannot use the current chemical drugs. The efficacy of existing drugs is limited. Although the stem cell treatment has recently emerged as a promising therapy for human diseases including lung fibrosis, the quality of stem cells cannot be controlled. The quality of stem cells is affected by many factors such as isolation, culture methods, and donors. Furthermore, stem cell transplant patients may face immune rejection and complications; its clinical efficacy was not significant compared with expectations. In Taiwan, the Ministry of Health and Welfare (MOHW) passed the amended "Regulations Governing the Application or Use of Specific Medical Techniques or Examinations, or Medical Devices". For the allowance scope of cell therapy programs, it mainly allows six areas of autologous cell therapy program while adopting strict review of allogeneic cells. It doesn’t include pulmonary fibrosis, so it is extremely difficult to apply stem cell treatment to the lung. Because cell therapy is complicated, labor-intensive, and personalized medical service, the cost of treatment is expensive. Based on the above reasons, there's currently no treatment that can stop or reverse the fibrosis of lungs. Peptides and proteins may have great potential as therapeutics to ease the plight of patients.
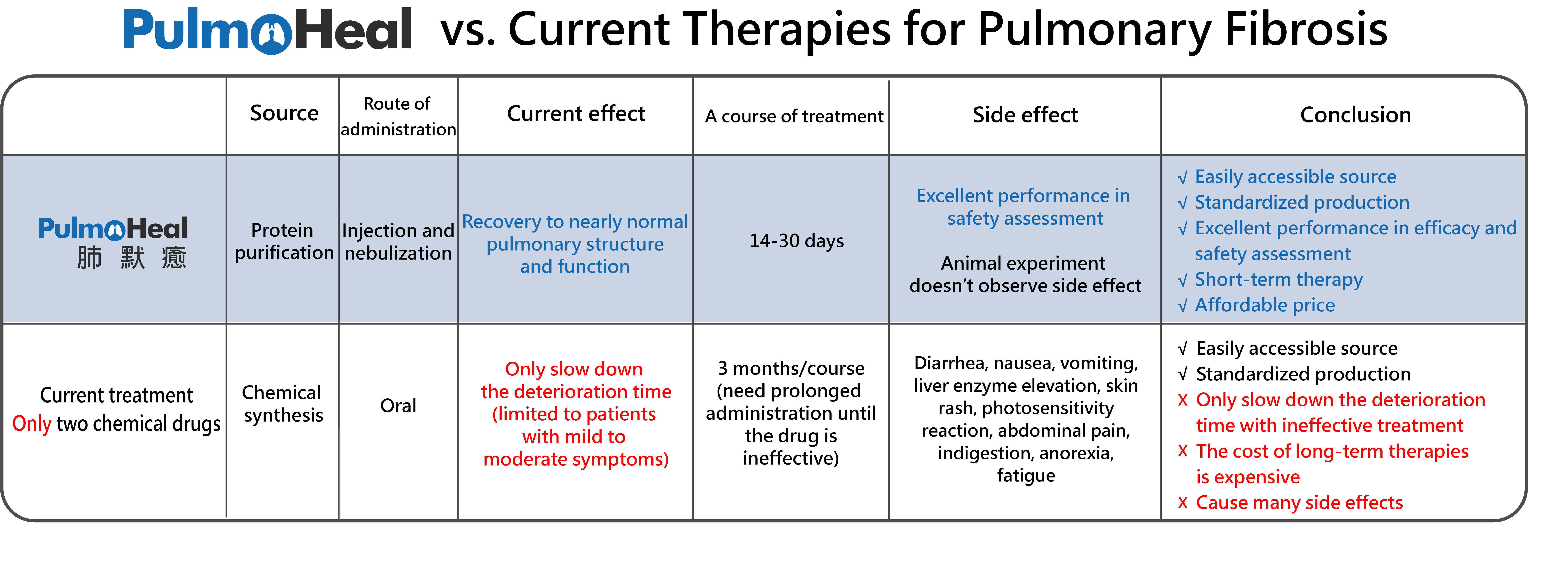
Compared with current or developing drug therapies for pulmonary fibrosis, the new protein drug – PulmoHeal® developed by Pulmo BioMed not only reverses the progression of pulmonary fibrosis but also helps pulmonary function and structure return to normal with short course of treatment. It indicates that PulmoHeal® may be a potential option for curing pulmonary fibrosis. Besides, the toxicological tests show that PulmoHeal® has excellent safety. It doesn’t cause serious side effects like extreme weight-loss and liver damage. In summary, PulmoHeal® has a strong developmental potential.
Link:JOYCOM Group
As many human diseases do not yet have appropriate medications, there is still lots of room for drug development. Dr. Chang leads the R&D team to invest in the field of drug research and development. The R&D group of Pulmo BioMed has been deeply working in the field of protein/peptide function for many years. Now we have successfully developed a new protein drug – PulmoHeal® with three advantages: (1) reversal of pulmonary fibrosis (2) short‐term therapy (3) high safety. PulmoHeal® can effectively reverse pulmonary fibrosis. The research and development achievements of PulmoHeal® were a breakthrough therapy in the treatment of pulmonary fibrosis. Because PulmoHeal® has a great development potential, we officially established Pulmo BioMed Co., Ltd. in 2021 to focus on recruiting funds and accelerating the acquisition of drug license. At the same time, we commit to promoting the listing of the company, optimize corporate governance levels and reinforce the management.
Many reasons cause pulmonary fibrosis, such as virus infection (COVID-19, SARS and other viruses), IPF, autoimmune disease (rheumatoid arthritis, lupus erythematosus, etc.), chronic inflammation, radiation therapy, cigarette smoking, air pollution and so on. Pulmonary fibrosis causes dyspnea and progressive deterioration of lung function in patients with a poor prognosis. Life expectancy of people with pulmonary fibrosis is generally less than five years. Worldwide, the incidence and prevalence of pulmonary fibrosis are increasing. Taking interstitial lung disease (ILD) for example, the prevalence of ILD are estimated 76.0 per 100,000 people in Europe and 74.3 per 100,000 people in the USA. According to research, patients with ILD have a high probability of developing pulmonary fibrosis. In addition, there are about 3 million people with IPF (a specific form of chronic, progressive fibrosing interstitial pneumonia) in the world and the number of patients is increasing rapidly. The global markets forecasting of IPF will reach a value of $8.8 billion by 2027. This reflects that the large patient population makes it become a hot treatment market and the demand is urgent. However, there is no effective cure for IPF. Currently, clinically approved drugs are chemical drugs which have long course of treatment and can only slow down the deterioration rate without curing effects. Patients with poor liver function must be monitored or even cannot use the current chemical drugs. The efficacy of existing drugs is limited. Although the stem cell treatment has recently emerged as a promising therapy for human diseases including lung fibrosis, the quality of stem cells cannot be controlled. The quality of stem cells is affected by many factors such as isolation, culture methods, and donors. Furthermore, stem cell transplant patients may face immune rejection and complications; its clinical efficacy was not significant compared with expectations. In Taiwan, the Ministry of Health and Welfare (MOHW) passed the amended "Regulations Governing the Application or Use of Specific Medical Techniques or Examinations, or Medical Devices". For the allowance scope of cell therapy programs, it mainly allows six areas of autologous cell therapy program while adopting strict review of allogeneic cells. It doesn’t include pulmonary fibrosis, so it is extremely difficult to apply stem cell treatment to the lung. Because cell therapy is complicated, labor-intensive, and personalized medical service, the cost of treatment is expensive. Based on the above reasons, there's currently no treatment that can stop or reverse the fibrosis of lungs. Peptides and proteins may have great potential as therapeutics to ease the plight of patients.
Compared with current or developing drug therapies for pulmonary fibrosis, the new protein drug – PulmoHeal® developed by Pulmo BioMed not only reverses the progression of pulmonary fibrosis but also helps pulmonary function and structure return to normal with short course of treatment. It indicates that PulmoHeal® may be a potential option for curing pulmonary fibrosis. Besides, the toxicological tests show that PulmoHeal® has excellent safety. It doesn’t cause serious side effects like extreme weight-loss and liver damage. In summary, PulmoHeal® has a strong developmental potential.